トピックス
第33回年会シンポジウム「薬物動態・毒性領域における新規評価ツールとその規制
(Novel drug developmental tools on DMPK/toxicity and their regulation)」
 |
Applicability of Micro Physiological Systems (MPS) to Drug Discovery and Development第一三共株式会社 薬物動態研究所
|
背景
新薬の開発には約10-15年の期間と26億米ドルという莫大な投資が必要であり,特に後期臨床試験における開発中止は臨床アンメットニーズに応える機会を失うことに加え,製薬企業にとっては経済的な打撃も大きい.1990年代において,臨床試験の主な失敗は経口バイオアベイラビリティ不足などの薬物動態的要因によるものであったが,その後はヒト肝臓ミクロソームを用いた代謝安定性試験やCaco-2細胞を用いた膜透過試験などのスクリーニング化により改善が見られ,現在では薬効および安全性が臨床試験における主な失敗要因となっている.
では,薬物動態面の改善は不要になったか?というと決してそのようなことはないと筆者は考える.我々薬物動態研究者は,従来の循環血中濃度をベースとした予測に加え,薬効ターゲット近傍および毒性発現組織における濃度推移,病態時の動態を精確に予測することで,さらなる成功確率の向上が期待される.既存のin vitro評価系は特定のintrinsicな反応をmimicすることは可能である一方,一部の機能についてはヒト応答を再現できていない.そこで期待される新たな技術がMicrophysiological System (MPS)である.
近年の微細加工技術および細胞工学の発展により,MPSはヒト生理応答を再現するin vitroツールとして注目され,2010年以降,欧米を中心に莫大な予算を投じた国家プロジェクトが複数進行している.中でも米国では2017年より『Tissue Chip 2.0』と銘打ち疾患モデル構築を含む3プロジェクトが産官学連携の下に展開され,多くのTissueモデルが構築され施設間バリデーションを含めたデータ取得フェーズに入った.これに並行して,大学からのスピンアウトを含めたMPS開発ベンダーが欧米中心に数多く誕生した.それらの多くは独自のサービスを展開する傍ら,製薬企業と1 on 1でがっちりと提携し,各社独自のニーズを満たすべくMPS開発に投資している.
MPS開発における重要因子
MPSの活用については創薬ステージに応じて使用目的・求められるスループットが異なる(図1)が,MPS開発における重要因子は①デバイス②細胞および③トランスレーショナル研究であると考える.
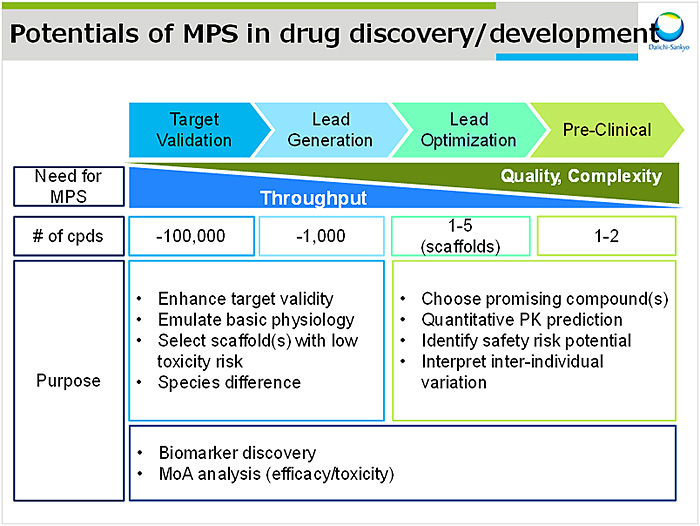
デバイスに関して用いる材質に加え,系の複雑さ及びスループットを考慮する必要がある.材質面では,①吸収・吸着といった化合物との相互作用が限りなく小さいものの選択,②界面に用いる膜素材(酸素透過が必要か),③検出系との相性,および④コストが重要である.系の複雑さに関して,MPSはチップ型とプレート型に大別される.チップ型は外部ポンプによる自由なフロー条件設定および伸縮を付加できるがスループットが低い.一方プレート型は外部ポンプを必要とせずシーソー型シェイカーを用いた重力駆動送液のため,省スペースかつスループットを稼ぐことができる.求めるニーズに応じてこれらを使い分けることが肝心である.
細胞については凍結細胞に加え新鮮細胞へのアクセスも改善され,iPS細胞,スフェロイド,オルガノイドと選択の幅が広がっている.さらにそれらに灌流培養やシアストレス(血液の粘性と血流の速度勾配に比例する物理力)を付加することで,従来の平面培養に比べて機能タンパクの発現および活性が大きく改善される例が多く報告されている.
トランスレーショナル研究については,MPSから得られたデータを如何にPBPKモデルに落としこめるかが鍵であり,Massachusetts Institute of TechnologyのDr. Griffithラボの開発した腸肝連結モデルを紹介した1).小腸部分はCaco-2,HT29細胞および樹状細胞を9:1:1で共培養し,肝臓部分は肝細胞およびクッパー細胞を10:1で共培養した.両チップを流路で連結し,ジクロフェナクのin vitro PK試験を行い,小腸および肝臓固有クリアランスを算出した.前述のとおりMPSデータの定量的な解析はまだ始まったばかりであるが,今後バリデートされた臓器モデルが構築されれば,多くのデータが蓄積されPBPKモデル構築につながると期待する.
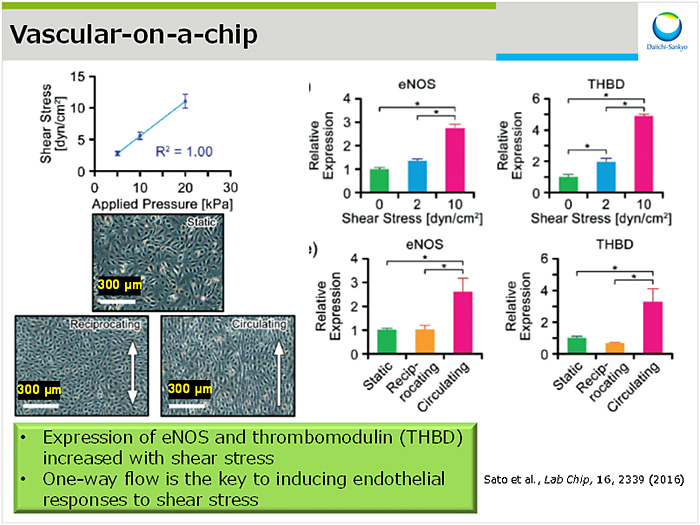
弊社での活用実例として,弊社が産業技術総合研究所との共同研究により構築した血管モデルについて紹介した(図2).シアストレス付加時の遺伝子発現変動をin vitro培養系で再現したいという薬理ニーズに応えるため,圧力駆動型ポンプによる流速依存的なストレスを負荷可能な血管チップを作成した2).本チップ上で灌流培養することで,シアストレス(0, 2, 10 dyn/cm2)依存的にeNOSやthrombomodulin発現上昇が認められた.
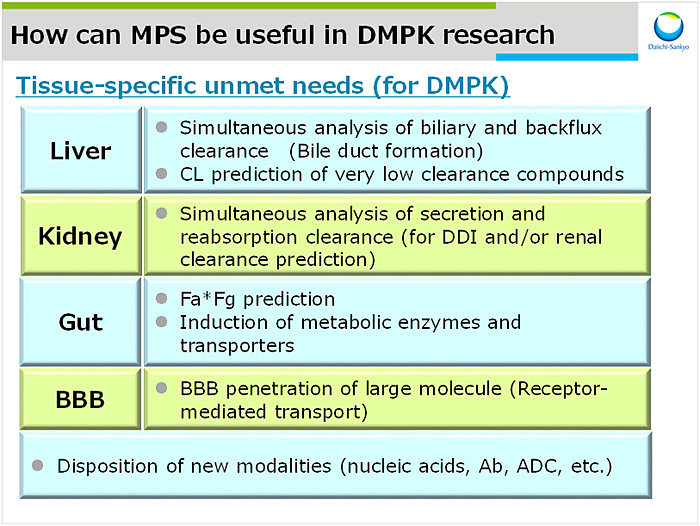
MPSの薬物動態分野への適用
MPSの薬物動態分野への適用について弊社ニーズ(図3)と報告事例を紹介した.University of WashingtonのDr. Kellyらは新鮮腎臓試料から近位尿細管細胞を単離培養し,独自のデバイス上でチューブ構造を形成することを見出した3).さらにこのモデルはパラアミノ馬尿酸の輸送がプロベネシドにより阻害されることからOAT活性の再現を示唆している.興味深いことに,本作用は同じ細胞を平面培養した際は観察されなかった.
Wyss Institute for Biologically Inspired Engineering at Harvard UniversityのDr. Ingberらはバイオプシーにより入手した新鮮小腸試料からクリプトを単離し上皮細胞に分化させ小腸内皮細胞と共培養させたモデルを報告している4).本モデルにおける遺伝子プロファイルを調べた結果,Transwell上で平面培養したCaco-2細胞のそれとは大きく異なり,十二指腸試料のそれと近い傾向を示した.本発表は薬物動態機能についての言及は少なかったが,期待度の高いモデルであると考えられる.
BBBモデルとして,SynVivo社が開発したSynBBBを紹介した5).既に市販されているチップ型デバイスであるが,HUVECとアストロサイトと共培養が可能(必要に応じてペリサイト)であり,P-gp基質であるRhodamin 123がcyclosporine Aおよびverapamil依存的に透過が促進することを報告している.
MPS活用にむけてのロードマップ
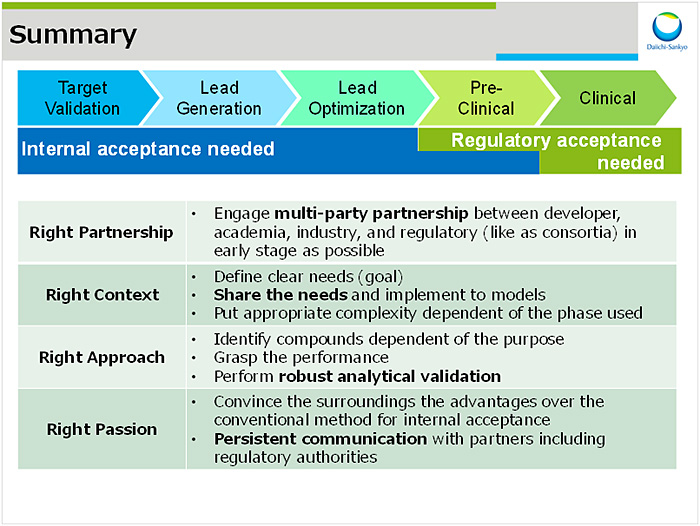
MPSを創薬におけるデファクトスタンダードとして活用するにあたって,①産官学の連携,②目的の明確化(およびそれに合致したシステム構築),③アウトプットの正しい解釈,④バリデーションが順次必要となる.これは2012年より開始した米国における第1期プロジェクトからのlessons & learnでもある.また,欧州動物代替学会の2015年における展望として,2020年には製薬企業においてMPSがメカニズム解析および一部の毒性試験に用いられるとされていたが,実際の創薬現場への適用は遅れている.これを加速化するためには,製薬企業のニーズをアカデミアおよびMPSベンダーと共有した上で迅速にモデルに落としこみ,バリデーションを実施すること,加えて規制当局とのコミュニケーションを含め多くのハードルを越えるという強い情熱が必要であると考えている(図4).
当日の質疑から
本発表に対して,ヒトでの外挿性を示す上での動物でのチップ作成の必要性,および創薬に活用する上でのコストの考え方について質問をいただいた.絞られた化合物についてMPSデータを社内decisionに用いる場合,動物でin vivo応答が認められるケースにおいては多少の工数が増えたとしても,動物チップによるin vitroからin vivoへの外挿性の確認は必要ではないかと考える.また,コストに関してはready-to-useタイプのデバイスは高価なケースが多く,一方,自社で培養条件の最適化を行うことは人的,時間的コストが大きい.このため,目的と緊急性を勘案して,ニーズ毎に総合的にMPS導入可否を判断する必要があるだろう.



